Specifications for Product Related Impurity Levels
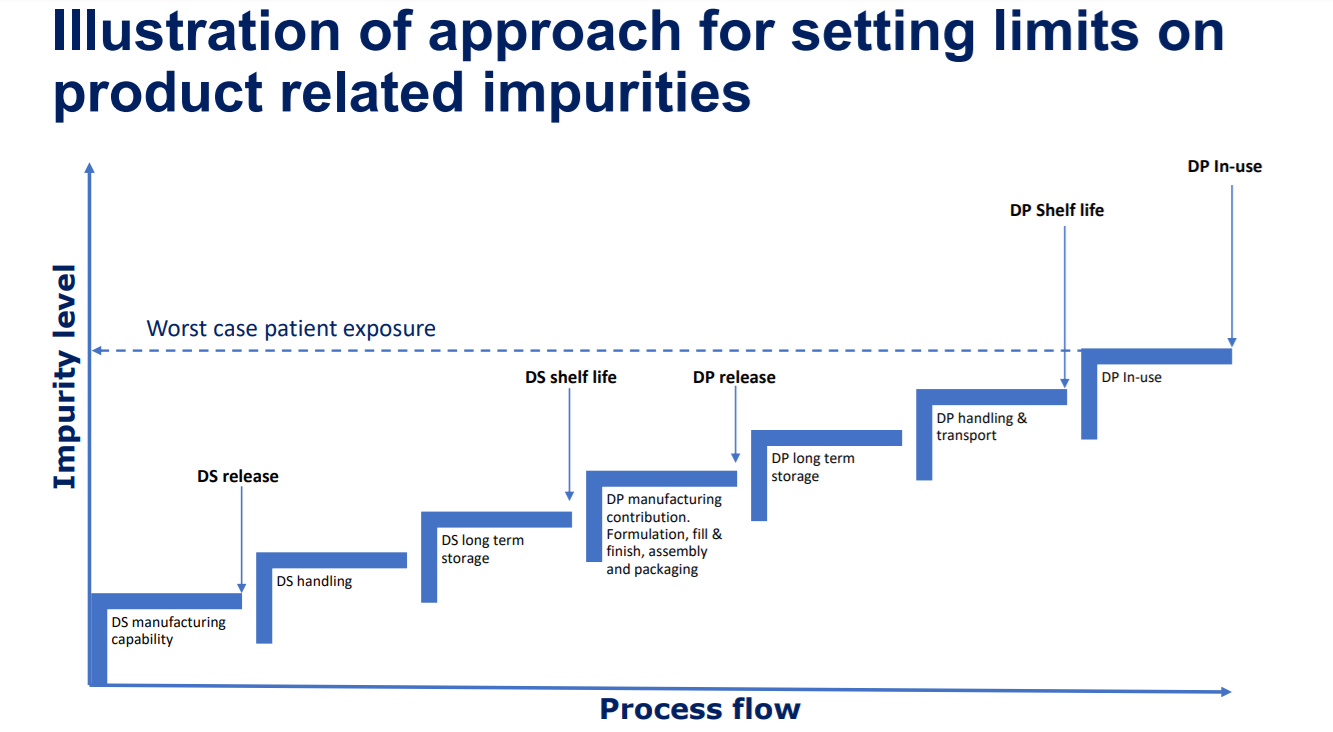
Defining commercial quality specifications for the product related impurity levels in injectable biologics can be a tricky affair. First we need to understand the contribution from the entire supply chain then we have to clinically qualify the worst case exposure level from a safety perspective. This work is interdiciplinary and requires tight collaboration between CMC and the safety/tox people throughout late stage development.
It is of key importance that the stability indicating parameters are monitored throughout development and the variables having an affect on the degradation products are well understood. Proper planning of the experimental work and stability program is of essence in order to ensure real-time data to support setting of the impurity limits.
The final impurity limits proposed for commercialization of the product must be adequately justified by a statistical data package and clinical safety qualification which is presented in the 3.2.P.5.6 section in the regulatory CTD for submission.
For more information on the topic see ICH guidelines Q3B, Q5C and Q6B.
In below image the potential increase in impurity levels throughout the supply chain is illustrated.