3.2.S.6 & 3.2.P.2.4: Extractables & Leachables
Introduction
The primary packaging of biologics should be conceived as an equally important design feature as the formulation itself. Apart from offering barrier and functional properties the container closure materials may leach compounds into the formulation which can affect quality, safety and efficacy. There are different routes from which impurities can arise from the packaging materials during storage and use:
· Migration of container closure compounds into the drug product
· Packaging material degradation and reaction products
· Volatile substances migrating into the drug product
This class of impurities are normally termed “leachables” or “migratable substances”.
Regulatory framework
Migratable substances may directly or indirectly impact the quality, efficacy, and hence the safety of finished drug products. Therefore, according to section 5.1 in the European Medicinal Agency CPMP/QWP/4359/03 Guideline on plastic immediate packaging materials (published 2005, section 5.1), studies to characterize and toxicologically evaluate the safety of the migratable substances in at least one representative batch of the drug product are generally expected to be performed as part of the development process.
The U.S. FDA Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics-Chemistry, Manufacturing, and Controls Documentation (published 1999, FDA link), do not specifically refer to migration studies but, for injectable drug products, the agency clearly expects an “extraction study on the packaging components to determine which chemical species may migrate into the dosage form (and at what concentration); and, secondarily, a toxicological evaluation”
How to characterize leachables?
When characterizing leachables, it is practical to define a concentration limit below which identification and toxicological evaluation is not performed. This involves two central concepts:
· Safety Concern Treshold (SCT)
A limit value based on compound toxicity (ug/day).
· Analytical Evaluation Threshold (AET)
A limit value based on SCT and daily dose of the product (ug/mL).
SCT’s for elemental impurities can be found in ICH Q3D.
The Product Quality Research Institute (PQRI) has published SCT’s for:
· Orally Inhaled and Nasal Drug Products (OINDP)
· Parental and Ophthalmic Drug Products (POPD)
SCT and AET should be included in section 3.2.S.6 and 3.2.P.7 of the module 3 sections of the regulatory dossier.
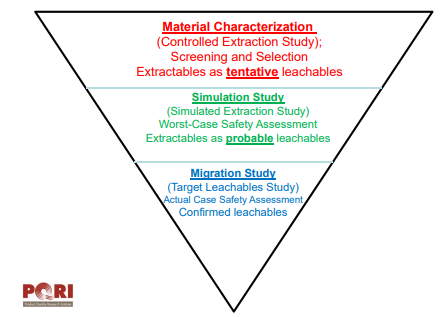
For the analysis of extractables and leachables, a three-step approach has been suggested by PQRI (illustrated in the picture below):
The 3-step approach suggested by PQRI to analyzing leachables.
The three steps involve the following:
1. Material characterization
Packaging material is subjected to accelerated conditions to identify extractable constituents (extractables).
Purpose: Approve or reject packaging candidate
2. Simulation study
Packaging material is subjected to solvent with similar extraction propensity as drug product
Purpose: Identify compounds for targeted leachables analysis in the drug product
3. Migration study
Targeted leachables study on aged drug product
Purpose: Evaluate whether packaging fulfills safety requirements under long term and in-use conditions
Leachables present above the SCT must be subjected to punctual toxicological evaluation and a Tolerated Exposure (TE) must be defined for the particular substance. Leachables must not exceed TE in the drug product.